Anode and Cathode in Electrolysis
An anode is an electrode where the oxidation reaction takes place or we can also define it as the terminal in which current flows into a device from outside. Anode is electron deficient and hence the negative ions are attracted to the anode where they lose electron and become atoms.
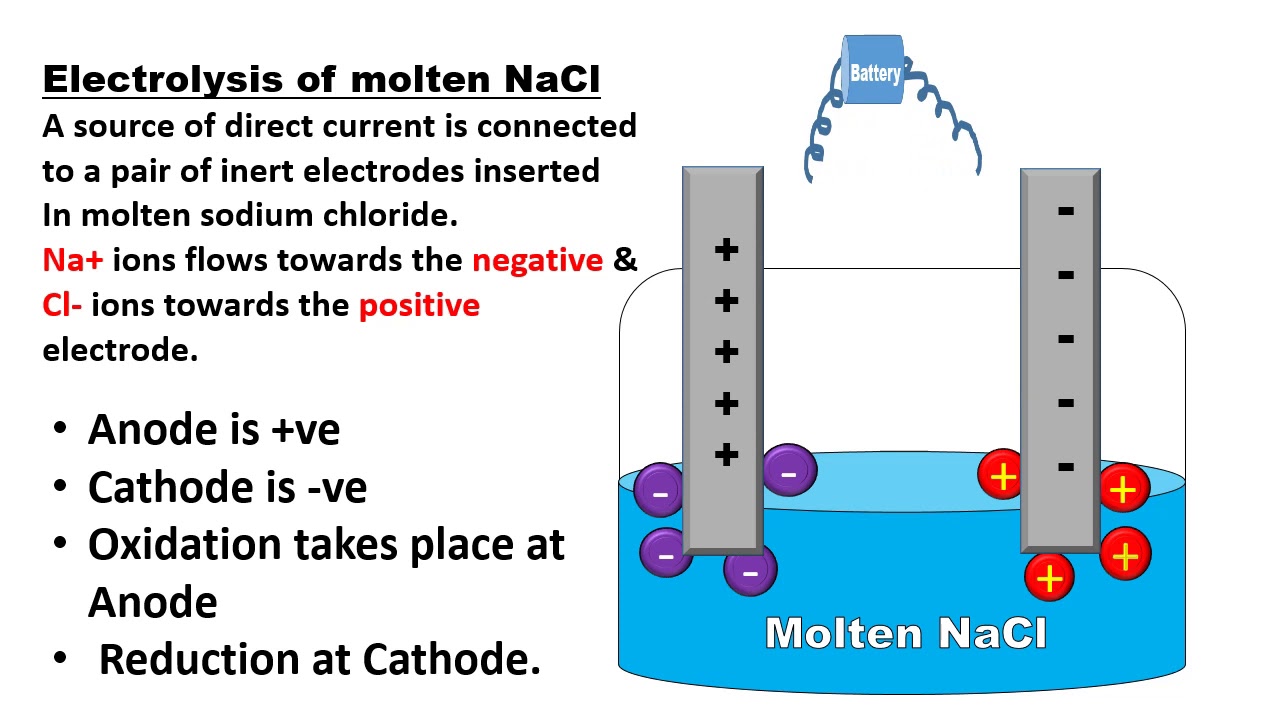
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
While reduction takes place at the cathode and the conventional current flows out of the device through this terminal.

. The electrode at which oxidation occurs is called an anode and the one at which reduction occurs is called the cathode whether it is an electrolytic cell or a galvanic cell. B During the electrolysis of copper II sulphate solution using platinum as cathode and carbon as anode i State what you observe at the cathode and at the anode ii State the change noticed in the electrolyte. It is the electrodemetal plate that is connected to the positive terminal of the cell.
Now about anode if cathode is negatively charged so it is easy to remember that anode is positively c. The battery pumps electrons away from the anode making it positive and into the cathode making it negative. Learn about the training of electrologists and the equipment needle-like probe used.
In chemistry a cathode is the electrode of an electrochemical cell at which reduction occurs. Springfield Massachusetts Dermatologist Doctors physician directory - Get information on the history of electrolysis a process for permanent hair removal. Since the anode can accept electrons oxidation occurs at that electrode.
The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment but also produces value-added materials on both sides. Name A - Z Sponsored Links. Answer 1 of 5.
The anode is the negative or reducing electrode from where electrons are released to the external circuit and oxidize during an. The cathode is negatively charged to remember how the cathode is negatively charged consider caThode T as a negative so it is easy to remember carhode as a negatively charged. These combined modifications gained reliability from the properties of tungsten controllable intensity from the ability to control the cathode filament temperature and controllable penetrating power from adjustment of the anode voltage.
Cathode leaves the electrode current and anode enters the current. The terms were coined in 1834 by William Whewell who derived the words from the Greek word Kathodes which means descent or way down. This marked a great advantage in medical diagnostics and assured.
In both kinds of electrochemical cells the anode is the electrode at which the oxidation half-reaction occurs and the cathode is the electrode at which the reduction half-reaction occurs. Anode mesh for seawater electrolysis We offers Platinized Titanium Anodes and MMO Anodes for Electrochemical and Metal Finishing Industries. Another mnemonic is to note the cathode has a c as does reduction.
Your Everlasting Solution Electrolysis by Cherie B. What is the difference between anode and cathode in electrolysis. The cathode is the current that leaves the electrodes or the cathode is a result of a reduction reaction taking place in an.
The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. A Galvanic cell converts chemical energy into electrical energy. In this article we will see the actual difference between anode and cathode.
What is the substance left at. Anode and Cathode. A useful mnemonic to remember this is AnOx RedCat Oxidation at the Anode Reduction at the Cathode.
The positive anode attracts anions toward it while the negative cathode attracts cations toward it. Solid round titanium bars coated with MMO are manufactured based on the life expectancy of the anodesCommon. 412 W Avon Rd.
Anode and cathode are defined by the flow of current. The electrolysis of acidulated water is considered to be an example of catalysis. Bichlor Electrolysers Out-perform The Closest Alternative Technology.
Cathode is the electrodemetal plate that is connected to the negative terminal of the cell. During electrolysis the anode loses mass as copper dissolves and the cathode gains mass as copper is deposited. Br ions lose electrons at the anode and become Br atoms which pair up to form Br 2 molecules.
The bombarded anode yielded the X-rays. Hence reduction at the cathode. Established in 1985 Jans Electrolysis is located at 45 Somers Rd in Hampden MA - Hampden County and is a business listed in the categories Electrolysis Treatments and Electrologists and offers Electrolysis Electrology and PERMANENT HAIR REMOVALAfter you do business with Jans Electrolysis please leave a review to help other.
For example in the electrolysis of water acidulated the half -reaction occurring at the anode is 2. A half-equation shows what happens at one of the electrodes during electrolysis. We supply MMO Tube anodes with KYNARHMWPEXLPEPVC cable attached as per customer requirements.
Pb 2 ions gain electrons at the cathode and become Pb atoms. The anode is the electrode where electricity moves into. The anode positive electrode is made from impure copper and the cathode negative electrode is made from pure copper.
To make efficient use of electrical energy in the whole electrocatalysis conversion process the integrating of anode and cathode reactions plays a vital role. Ad World-class Electrolysers And Electrode Coatings That Produce Chlor-alkali Products. The cathode is the electrode where electricity is given out or flows out of.
What is anode and cathode in electrolysis. Answer 1 of 3. Southern Connecticut Electrolysis in Springfield MA.

Electrochemistry Featuring Electrolysis And Fuel Cells Chemistry Classroom Electrochemistry Chemistry Lessons

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Piscinas Escuela De Natacion

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions

Look4chemistry Electrolytic Cell Electrochemistry Cell Redox Reactions
0 Response to "Anode and Cathode in Electrolysis"
Post a Comment